Coenzyme Definition
A coenzyme is an organic non-protein compound that binds with an enzyme to catalyze a reaction. Coenzymes are often broadly called cofactors, but they are chemically different. A coenzyme cannot function alone, but can be reused several times when paired with an enzyme.
Functions of Coenzymes
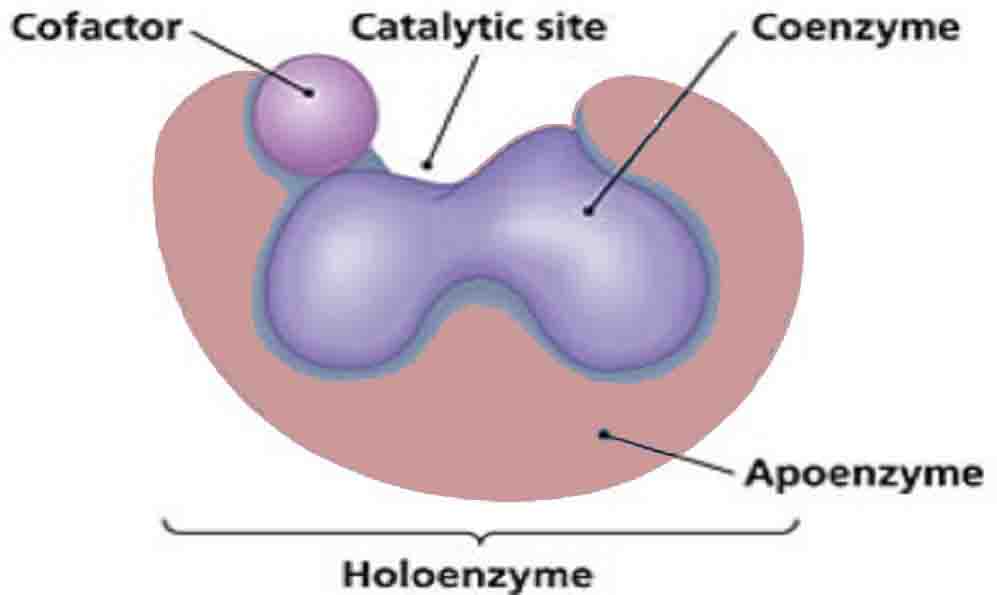
An enzyme without a coenzyme is called an apoenzyme. Without coenzymes or cofactors, enzymes cannot catalyze reactions effectively. In fact, the enzyme may not function at all. If reactions cannot occur at the normal catalyzed rate, then an organism will have difficulty sustaining life.
When an enzyme gains a coenzyme, it then becomes a holoenzyme, or active enzyme. Active enzymes change substrates into the products an organism needs to carry out essential functions, whether chemical or physiological. Coenzymes, like enzymes, can be reused and recycled without changing reaction rate or effectiveness.
They attach to a portion of the active site on an enzyme, which enables the catalyzed reaction to occur. When an enzyme is denatured by extreme temperature or pH, the coenzyme can no longer attach to the active site.
Types of Enzymes
Cofactors are molecules that attach to an enzyme during chemical reactions. In general, all compounds that help enzymes are called cofactors. However, cofactors can be broken down into three subgroups based on chemical makeup and function:
Coenzymes
These are reusable non-protein molecules that contain carbon (organic). They bind loosely to an enzyme at the active site to help catalyze reactions. Most are vitamins, vitamin derivatives, or form from nucleotides.
Cofactors
Unlike coenzymes, true cofactors are reusable non-protein molecules that do not contain carbon (inorganic). Usually, cofactors are metal ions such as iron, zinc, cobalt, and copper that loosely bind to an enzyme’s active site. They must also be supplemented in the diet as most organisms do not naturally synthesize metal ions.
Prosthetic groups
These can be organic vitamins, sugars, lipids, or inorganic metal ions. However, unlike coenzymes or cofactors, these groups bind very tightly or covalently to an enzyme to aid in catalyzing reactions. These groups are often used in cellular respiration and photosynthesis.
Examples of Coenzymes
Most organisms cannot produce coenzymes naturally in large enough quantities to be effective. Instead, they are introduced to an organism in two ways:
Vitamins
Many coenzymes, though not all, are vitamins or derived from vitamins. If vitamin intake is too low, then an organism will not have the coenzymes needed to catalyze reactions. Water-soluble vitamins, which include all B complex vitamins and vitamin C, lead to the production of coenzymes. Two of the most important and widespread vitamin-derived coenzymes are nicotinamide adenine dinucleotide (NAD) and coenzyme A.
NAD is derived from vitamin B3 and functions as one of the most important coenzymes in a cell when turned into its two alternate forms. When NAD loses an electron, the low energy coenzyme called NAD+ is formed. When NAD gains an electron, a high-energy coenzyme called NADH is formed.
NAD+ primarily transfers electrons needed for redox reactions, especially those involved in parts of the citric acid cycle (TAC). TAC results in other coenzymes, such as ATP. If an organism has a NAD+ deficiency, then mitochondria become less functional and provide less energy for cell functions.
When NAD+ gains electrons through a redox reaction, NADH is formed. NADH, often called coenzyme 1, has numerous functions. In fact, it is considered the number one coenzyme in the human body because it is necessary for so many different things. This coenzyme primarily carries electrons for reactions and produces energy from food.
For example, the electron transport chain can only begin with the delivery of electrons from NADH. A lack of NADH causes energy deficits in cells, resulting in widespread fatigue. Additionally, this coenzyme is recognized as the most powerful biological antioxidant for protecting cells against harmful or damaging substances.
Coenzyme A, also known as acetyl-CoA, naturally derives from vitamin B5. This coenzyme has several different functions. First, it is responsible for initiating fatty acid production within cells. Fatty acids form the phospholipid bilayer that comprises the cell membrane, a feature necessary for life. Coenzyme A also initiates the citric acid cycle, resulting in the production of ATP.
Non-Vitamins
Non-vitamin coenzymes typically aid in chemical transfer for enzymes. They ensure physiological functions, like blood clotting and metabolism, occur in an organism. These coenzymes can be produced from nucleotides such as adenosine, uracil, guanine, or inosine.
Adenosine triphosphate (ATP) is an example of an essential non-vitamin coenzyme.
In fact, it is the most widely distributed coenzyme in the human body. It transports substances and supplies energy needed for necessary chemical reactions and muscle contraction. To do this, ATP carries both a phosphate and energy to various locations within a cell. When the phosphate is removed, the energy is also released.
This process is result of the electron transport chain. Without the coenzyme ATP, there would be little energy available at the cellular level and normal life functions could not occur.
Related Biology Terms
- Catalyze – To cause or accelerate a reaction.
- Enzyme – A protein that catalyzes chemical reactions within an organism.
- Active Site – The region on an enzyme where substrates bind during a reaction.
- Substrate – The substance on which an enzyme acts to make a new product.